
LifeTech Scientific Corporation was established in Shenzhen, China


HeartR? PDA Occluder was approved by NMPA in China, becoming the first product successfully marketed by the company


HeartR? ASD Occluder was approved by NMPA in China

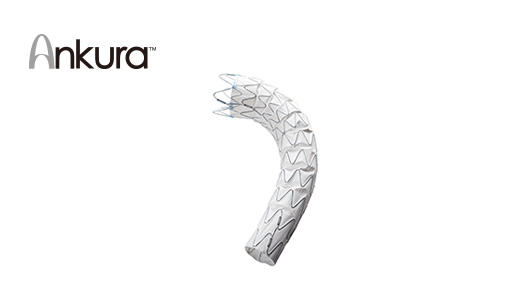
Ankura? TAA\AAA Stent Graft System and HeartR? VSD Occluder were approved by NMPA in China


Aegisy? Vena Cava Filter was approved by NMPA in China

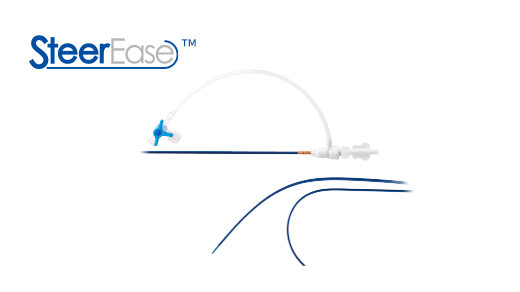
SteerEase? Introducer was approved by NMPA in China


The company was accredited as a national high-tech enterprise in China
The company expanded its business into India market and incorporated Lifetech Scientific India Private Limited into the LifeTech family

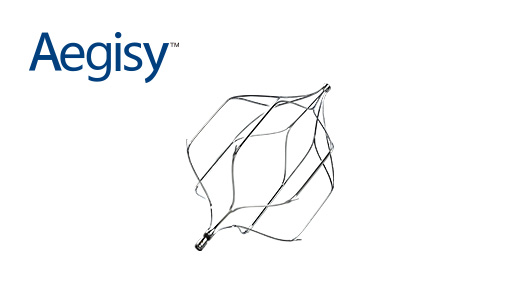
Aegisy? Vena Cava Filter obtained the CE mark in EU, becoming the first product of the company to receive one
Cera? Occluder series and SteerEase? Introducer obtained the CE mark in EU


SeQure? Snare System received FDA approval in the United States, becoming the first product of the company to receive one
Fustar? Steerable Introducer, Cera? Vascular Plug System, Acumark? Sizing Balloon, Cera? Multi-Fenestrated ASD Occluder and SeQure? Snare System obtained the CE mark in EU


LifeTech Scientific Corporation was listed on the Hong Kong Stock Exchange (Stock Code: 1302.HK)
The company incorporated LifeTech Scientific (Europe) Co?peratief U.A. to further explore the European market
Fustar? Steerable Introducer received FDA approval in the United States
CeraFlex? Occluder series and Vascular Plugs, Ankura? Stent Graft System obtained the CE mark in EU


The company incorporated LifeTech Scientific (Netherlands) B.V. into the LifeTech family
Cera? PDA Occluder and ASD Occluder were approved by NMPA in China
HeartR? Occluder series, Cera?/CeraFlex?/IrisFit? PFO Occluders and LawMax? Dilator obtained the CE mark in EU


Cera? VSD Occluder was approved by NMPA in China
LawMax? Dilator received FDA approval in the United States


Fustar? Steerable Introducer was approved by NMPA in China


The company has been included in the Hang Seng Index
The company incorporated Lifetech Scientific America Corporation to add to its list of international subsidiaries


The company has been included in the Shenzhen-Hong Kong Stock Connect Programs
The company established a subsidary in Hong Kong and Greece, respectively
LAmbre? LAA Closure System and GoldenFlow? Peripheral Bare Stent System obtained the CE mark in EU
SteerNavi? Introducer was approved by NMPA in China


LAmbre? LAA Closure System, HeartTone? Implantable Cardiac Pacemakers, SeQure? Snare System, LawMax? Dilator and Ankura? II Stent Graft System were approved by NMPA in China


The company established a strategic partnership with Ally Bridge Group, a global life science-focused investment firm
KONAR-MF? VSD Occluder obtained the CE mark in EU


The company incorporated LifeTech Scientific Deutschland GmbH to further expand its business in the heart of Europe
LAmbre? LAA Closure System obtained FDA approval for the pre-market clinical study in the United States
Aelark? Vena Cava Filter System was approved by NMPA in China


LAmbre? LAA Closure System obtained FDA approval for an investigator-initiated pre-market clinical trial in the United States
HeartR? II ASD Occluder, Fustar? mini Steerable Catheter, FiQure? Vena Cava Filter Retrieval System, ZoeTrack? Guidewire, FemCross? 18 Peripheral Balloon Dilatation Catheter, Futhrough? Stent Graft Balloon Catheter were approved by NMPA in China
Futhrough? Endovascular Needle System obtained the CE mark in EU


The invention patent ŌĆ£LAA OccluderŌĆØ won the WIPO-CNIPA Award for Chinese Outstanding Patented Invention
G-iliac? Iliac Artery Bifurcation Stent Graft System, Yuranos? Abdominal Aortic Stent Graft System, LAxible? LAA Occluder, LAnavi? Jointed Steerable Introducer, OKcurve? Steerable Delivery System, Freepath? Guidance System and iCable? Delivery Cable were approved by NMPA in China
IBS Angel? Iron Bioresorbable Scaffold System obtained registration approval from Malaysian Medical Device Authority (MDA)
Xuper? Open Surgery Stent Graft System obtained the CE mark in EU


An FDA-approved investigator-initiated pre-market clinical trial of LAmbre? Plus LAA Closure System obtained medical insurance coverage in the United States
Fitaya? Vena Cava Filter System, FemCross? 35 Peripheral Balloon Dilatation Catheter and Ankura? llc Stent Graft System were approved by NMPA in China

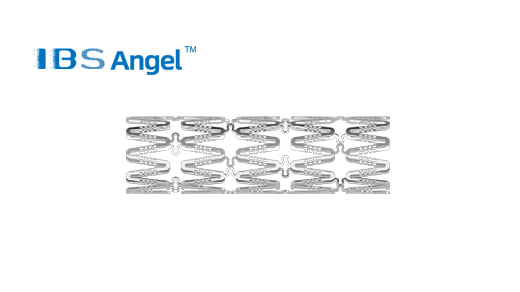
IBS Angel??Iron Bioresorbable Scaffold System obtained CE MDR certification in EU
Peripheral?Thrombus?Aspiration?Catheter, AcuMark? Sizing Balloon and Epione??Surgical?Navigation?System were approved by NMPA in China


Futhrough? Endovascular Needle System, Thrombectomy Aspiration Pump, Balloon Guided Catheter, Distal Access Catheter Kits, Intracranial Aspiration Catheter and HeartTone? Implantable Cardiac Pacemaker compatible with magnetic resonance imaging (ŌĆ£MRIŌĆØ) were approved by NMPA in China.


Strategic investment in Affector Medtech (Suzhou) Ltd. to expand presence in the field of cardiac electrophysiology.
Aortic Stent Graft System (consists of the Ankura? Pro Aortic Stent Graft System and Longuette? Aortic Branch Stent Graft System), Aortic Arch Stent Graft System (consists of the Ankura? Plus Aortic Arch Stent Graft System and CSkir? Aortic Arch Branch Stent Graft System), Peripheral Balloon Dilatation Catheter (Large diameter), Yoscop? Multi-loop Snare System and SteerEase?-m Introducer were approved by NMPA in China.


